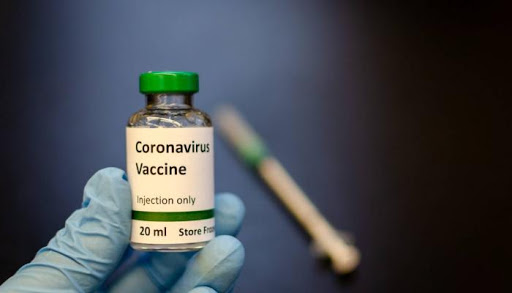
Trials involving 1,077 individuals showed the injection led to them creating antibodies and white blood cells which will fight coronavirus. The Oxford vaccine — referred to as ChAdOx1 of-19 — is created from a harmless chimpanzee virus. The human vaccine trial has been developed by scientists at Oxford University`s Jenner Institute.
One of the vaccine candidates acquired at the University of Oxford has shown encouraging ends up in early human testing and seems to be “safe well-tolerated, and immunogenic”, the findings of the trial revealed within the Lancet journal aforementioned.
Trials involving 1,077 individuals showed the injection led to them creating antibodies and white blood cells which will fight coronavirus.
Our introductory decisions show that the candidate vaccine given as one dose was safe and tolerated, despite a better reactogenicity outline than the control vaccine, MenACWY, stated the researchers, which they also wrote within the study created for the same reason.
“No serious adverse reactions to ChAdOx1 nCoV-19 occurred. The bulk of adverse events reported were gentle or moderate in severity, and all were self-limiting,” the study aforementioned.
The clinical trials of the potential COVID-19 vaccine on humans were conducted between April 23 and May 21.
The Oxford vaccine — referred to as ChAdOx1 of-19 — is created from a harmless chimpanzee virus. The human vaccine trial has been developed by scientists at Oxford University`s Jenner Institute.
According to the findings of the study, 1077 participants were listed and assigned to receive either ChAdOx1 to-19, 10 of whom were listed in the non-randomized ChAdOx1 to-19 prime-boost group.
Local and general reactions were more common within the ChAdOx1 nCoV-19 group and many were reduced by the employment of prophylactic paracetamol, together with pain, feeling feverish, chills, muscle ache, headache, and malaise). There have been no serious adverse events regarding ChAdOx1 to-19. Within the ChAdOx1 nCoV-19 group, spike-specific T-cell responses peaked on day 14. Anti-spike responses issued by day 28, and were raised following a second dose, the study aforementioned.
Counterbalancing antibody answers against SARS-CoV-2 were identified in 32 (91%) of 35 participants once a single dose when measured in MNA80 and in 35 (100%) participants when measured in PRNT50. After a booster shot, all participants had neutralizing activity (nine of 9 in MNA80 at day 42 and 10 of ten in Marburg VN on day 56). Neutralizing antibody responses related to strongly with antibody levels measured by Elisa, it added.